New details on the formation of 'metal soaps' in oil paint that hinder the conservation of artworks
Mar 05, 2022by National Institute of Standards and Technology

When you think of soaps, you may first picture the liquid foams or solid bars that wash away dirt, grime and bacteria. However, not all soaps are cleansers. In the art conservation world, metal carboxylates, also known as "metal soaps," are undesirable: They form in ongoing chemical reactions that can damage the integrity of the paint and the appearance of paintings over time.
Though these compounds have long been found in oil paintings, surprisingly little is known about how they form and then proceed to damage these works of art. Now, researchers from the National Institute of Standards and Technology (NIST) have collaborated with the National Gallery of Art and other institutions to use novel infrared-light-based methods that identify the composition and distribution of these metal soaps at multiple levels of detail. Their findings may ultimately help art conservators better preserve oil paintings.
The researchers have published their findings in Analytical Chemistry.
Oil paintings don't appear to change in front of the naked eye. However, once the paint dries, it's not set in stone. Driven by environmental factors such as humidity and temperature, chemical reactions at the microscopic level constantly occur among the oils and pigments. Over time, whether a few years or centuries, the resulting metal soaps can cause paint degradation, such as cracking or peeling of paint layers.
Like bath soaps, which are composed of fatty acid molecules bonded to sodium ions, metal soaps are composed of fatty acid molecules, formed when the oils in paint react with water and oxygen, and bond to metal ions leached out from the pigments. Paints with zinc and lead pigments frequently form metal soaps, while many other metal-containing paints (such as aluminum, calcium and manganese) have been reported to form soaps as well.
In the study, researchers analyzed a French 19th-century painting, "Gypsy Woman with Mandolin," by artist Jean-Baptiste-Camille Corot, which is in the collection of the National Gallery of Art in Washington, D.C.
"The painting had some problems that art conservators pointed out," said NIST researcher Andrea Centrone. "It has 13 layers, many due to restorations that occurred long after the painting was made, and at the very least the top layer was degrading. They wanted to restore the painting to its original state of appearance and to find out what was happening on a microscopic level on the top layer of the painting; and that's where we started to help."
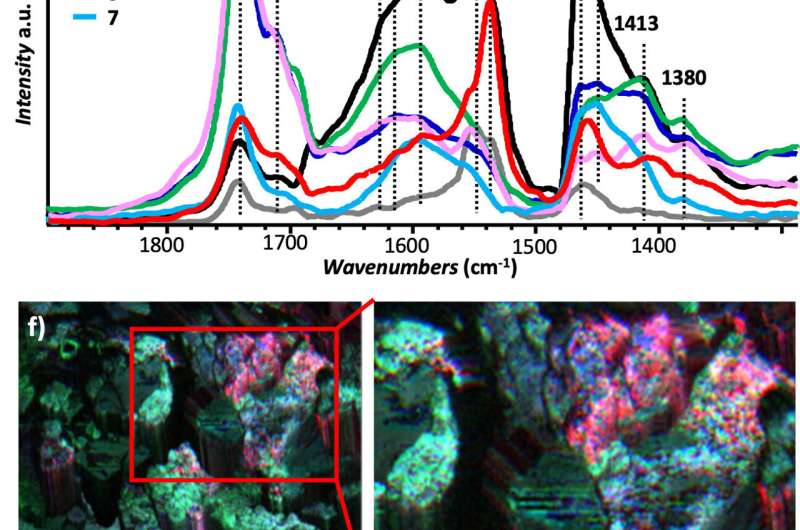
To minimize damage to the painting, researchers took a small sample from a degraded area using a surgical scalpel. The composition of the top layer was analyzed, and they quickly determined that the paint consisted of dried oil, cobalt green and lead white pigments, and metal soaps. They then characterized the metal soaps by using three separate infrared light techniques that revealed details from the scale of micrometers (millionths of a meter) to nanometers (billionths of a meter).
The first method, known as infrared microscopy, is a well-established technique that is often used to identify a sample's chemical composition because the specific wavelengths (colors) of infrared light absorbed by a material or by a molecule are equivalent to a fingerprint.
Using state-of-the-art infrared microscopy, which has spatial resolution at the level of millionths of a meter, it was possible to identify the general presence of metal soaps, but not the specific individual types.
"A fundamental limit of optical microscopy is that light cannot be focused to a spot smaller than half its wavelength," said Centrone. "Infrared light has wavelengths between 2 and 20 micrometers, and although it sounds small, it is too big for measuring details with nanometer-scale spatial resolution."
As the researchers learned over the course of their work, nanoscale spatial resolution is critical for resolving how the paints' original components and their alteration byproducts are intermixed, which is necessary to better understand chemical reactions in paints.
To see tinier details, the researchers used two methods that rely on "tricks" to bypass the usual resolution limit. At the laboratory of Photothermal Spectroscopy Corporation in Santa Barbara, California, they used a technique known as optical photothermal infrared spectroscopy (O-PTIR).
In O-PTIR, light from a pulsed infrared laser and a constant green laser are both focused on the sample. The infrared light pulses, cycling through a series of different infrared wavelengths, heat up the sample. The heated sample reflects an amount of green laser light that depends on how much infrared light it absorbs at each wavelength.
Since the wavelength of the green laser (532 nanometers) is much smaller than the infrared wavelengths, it can be focused to a much smaller spot and effectively provide infrared spectra, and therefore details on the sample's chemical composition, with a resolution of about 500 nanometers (billionths of a meter).
"The wavelengths absorbed by the sample are like fingerprints that can be used to identify specific chemical compounds," said Centrone. Using this technique, researchers discovered that a variety of zinc soaps, but not lead soaps, were present in the sample. They identified specific species of zinc soaps such as zinc stearate and zinc oleate, which are dense and ordered (crystalline) metal soaps, as well as a pervasively disordered (amorphous) zinc soap.
Because of the amorphous zinc soap's disordered structure, researchers think it allows the slow transport or diffusion of water, ions and other chemical compounds, which may then react, facilitating the formation and growth of metal soap clusters over time. Still the spatial resolution was insufficient to see how the three types of metal soaps were distributed in the sample.
To sharpen their view, the researchers used a technique known as photothermal induced resonance (PTIR) at NIST's campus in Gaithersburg, Maryland. In PTIR, light from a pulsed infrared laser (also having a range of wavelengths) heats up the sample, which expands and contracts very quickly after each pulse. The sample pulsates against the tip of an atomic force microscope cantilever, which begins to vibrate like a struck tuning fork. Researchers measured the extent of the cantilever vibration to determine how much light was absorbed by the sample.
This way, PTIR provides chemical maps and IR spectra with a resolution of 10 to 20 nanometers by scanning the tip over the sample surface. "PTIR spatial resolution brings into view chemical composition details not obtainable before. You can see how the oils and the metal soaps in the paints are distributed. There is a lot of variation at the nanoscale," said Centrone.
While PTIR provides the highest spatial resolution, it takes more time to map the sample area because of the very small (10 nm by 10 nm) size of the pixels it produces to build an image. O-PTIR enables the rapid identification of chemical compounds on a 500 nm scale, while conventional infrared microscopes are most useful to assess changes in paint composition over large areas. Such a multiscale approach could help art conservators identify and assess the impact of the different species of metal soaps, and better understand how they come about and how they interact within paint.
The technique can also help provide strategies to conserve oil paintings, such as determining which pigments are prone to degradation by reacting and forming metal soaps. "Characterization of the different sorts of metal carboxylates in oil paint will help to understand the initiating factors for the detrimental kinds of soap formation and aggregation, and will help art conservators develop new preservation strategies," said Barbara Berrie, head of science conservation and senior conservation scientist at the National Gallery of Art.
This is a blatant attempt to get you to sign up.
And if you do, I promise not to be too boring or pedantic. So if you want, fill in the form below.
I hate SPAM, so I won't never ever sell your information, for any reason.

